SmPC Compliance – Getting Product Information Right
- Aug 8, 2025
- 6 min read
Updated: Sep 5, 2025
Table of Contents
Introduction to The Summary of Product Characteristics (SmPC)
The SmPC is a critical, scientific, regulator-approved document that defines every authorised medicine’s benefits, risks and conditions of use.
The SmPC is synonymous with the South African Professional Information (PI) leaflet which is regulated in terms of Regulation 11 of Act 101/1965 (The Medicines and Related Substances Act).
The SmPC follows the format stipulated by the European Medicines Agency (EMA) Directive 2001/83/EC (2025).

What is an SmPC and Why Does Compliance Matter?
The SmPC is a critical (and legal) part of the registration application for every medicine. It is an integral part of the registration application and is presented in Module 1.3. of the Common Technical Document (the CTD). The SmPC must be aligned and referenced against CTD Modules 2 -5 and SAHPRA’s Guidelines.
The SmPC is subject to review and registration approval by SAHPRA. It serves as the master document upon which patient leaflets and promotional claims are based. Non-compliant SmPCs will result in regulatory findings or even rejection of the medicine’s application.
Global Regulatory Framework
South Africa & SADC & the WHO
SAHPRA adopts a tripartite model: SmPC (called the package insert), patient leaflet and outer labelling, aligned with a 13-section structure derived from EU rules. (sahpra.org.za). The WHO promotes reliance, harmonisation, and SmPC alignment across regions like SADC, EAC, and ZAZIBONA
The European Union
Directive 2001/83/EC (Article 11) lists the mandatory headings and legal status of the SmPC. (eur-lex.europa.eu)
EMA’s Quality Review of Documents (QRD) template sets standard wording
; v11 is now in public consultation (deadline 31 Aug 2025).
The upcoming ePI system (electronic Product Information) is a digital, standardised version of the SmPC, PIL (Patient Information Leaflet), and labelling—structured in machine-readable XML format instead of traditional PDFs or Word files.
This XML format will allow for:
Searchable and filterable content
Integration into electronic health records (EHRs)
Automated updates
Accessibility for mobile and web applications
SAHPRA has not yet aligned to the ePI, and may adopt similar digital standards over time.
The United Kingdom
The MHRA retains the EU QRD headings but applies its own labelling and packaging best-practice guidance post-Brexit. (gov.uk, assets.publishing.service.gov.uk)
Approved UK SmPCs are published on the MHRA Product Information Portal.
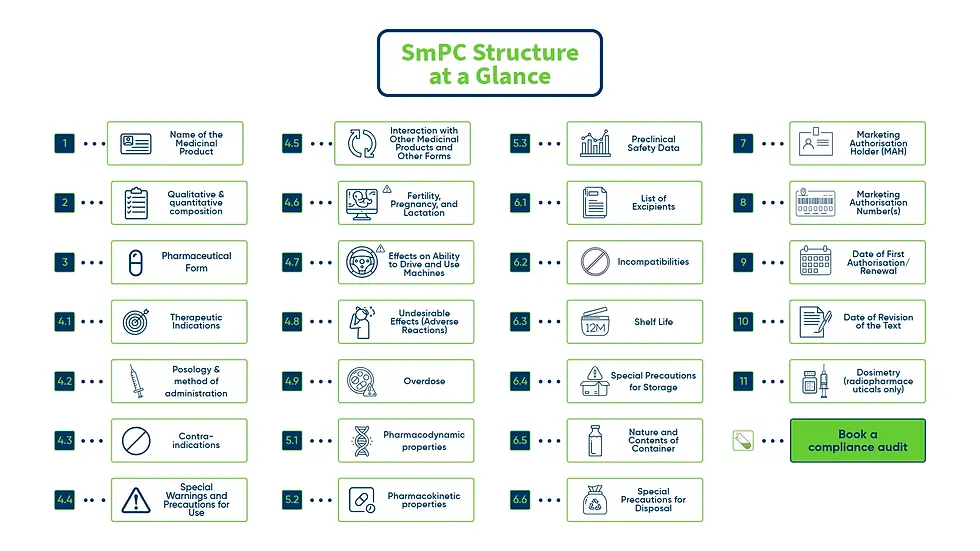
SmPC Structure at a Glance
The SmPC Structure is based on the EMA QRD template, widely adopted and adapted by SAHPRA and many WHO-aligned regulatory authorities:
Section | Title | Purpose |
“Scheduling Status” to appear above Section 1 | ||
1 | Name of the Medicinal Product | Name, strength & dosage form |
2 | Qualitative and Quantitative Composition | API content & excipients of known effect |
3 | Pharmaceutical Form | Description (e.g., tablet, solution) & appearance |
4.1 | Therapeutic Indications | Approved medical uses |
4.2 | Posology and Method of Administration | Dosing regimen & administration instructions |
4.3 | Contraindications | Conditions or factors that prohibit use |
4.4 | Special Warnings and Precautions for Use | Key safety risks and precautions |
4.5 | Interaction with Other Medicinal Products and Other Forms | Known or expected drug interactions |
4.6 | Fertility, Pregnancy, and Lactation | Use considerations in these groups |
4.7 | Effects on Ability to Drive and Use Machines | Safety information regarding psychomotor skills |
4.8 | Undesirable Effects (Adverse Reactions) | Side effects with frequencies |
4.9 | Overdose | Signs, symptoms, and management |
5.1 | Pharmacodynamic Properties | Mechanism of action & therapeutic class |
5.2 | Pharmacokinetic Properties | ADME profile (Absorption, Distribution, Metabolism, Excretion) |
5.3 | Preclinical Safety Data | Toxicology and safety in animals |
6.1 | List of Excipients | All non-active ingredients |
6.2 | Incompatibilities | Known incompatibilities with other substances |
6.3 | Shelf Life | Duration of product stability |
6.4 | Special Precautions for Storage | Storage conditions (e.g., refrigeration) |
6.5 | Nature and Contents of Container | Packaging material and configuration |
6.6 | Special Precautions for Disposal | Safe disposal and handling instructions |
7 | Marketing Authorisation Holder (MAH) | Name and address |
8 | Marketing Authorisation Number(s) | Regulatory reference numbers |
9 | Date of First Authorisation/Renewal | Historical regulatory data |
10 | Date of Revision of the Text | Last update to SmPC |
11 | Dosimetry (radiopharmaceuticals only) | Radiation exposure details |
Compliance Life-Cycle
SmPC (or Professional Information/PI in SAHPRA terms) compliance is not static—it's a life-cycle obligation that spans from product development to post-marketing. Here’s a structured view of the key compliance aspects throughout the SmPC life-cycle:
Initial Registration & Approval
SmPC must be technically validated and complete at submission.
SAHPRA requires the SmPC (PI) to form the basis for the PIL.
Scientific, unambiguous, and non-promotional language is mandatory.
Excipient listings, shelf life, dosage forms, and manufacturer details must match other dossier modules.
Post-Registration Maintenance (Variations & Updates)
SmPC must be updated to reflect new safety data (e.g. pharmacovigilance, clinical literature).
All clinical and safety updates (new indications, contraindications, adverse effects) require Type II variation applications.
For reliance-based submissions, SmPC updates must align with recognised reference authorities.
Renewals & Retention
Renewal submissions must confirm that the SmPC remains current and accurate.
Retention Requirements
Retention of registration every five years includes compliance check of SmPC accuracy and consistency.
Pharmacovigilance-Linked Obligations
Adverse event trends must trigger updates in SmPC Section 4.8 (Undesirable Effects).
Risk Management Plans (RMPs)
Must inform and align with SmPC content (e.g. Section 4.4: Warnings and Precautions).
Frequent Deficiencies & How to Avoid Them
Deficiency | Possible Root Cause | Fix |
Safety class warnings missing | Copy/paste from originator PI without updates or incorrect references used | Requires a detailed review and a line-by-line gap analysis against the reference SmPC. |
Mis-aligned SPC/PIL text | Different persons working of the document and no final document checks or validation. | Maintain single‐source master documents and references in RIM tools |
Cross-referenced non-existing studies | Poor document control | List only peer-reviewed or regulator-accepted references. |
MC Pharma’s SmPC Compliance Services
Medical writing & SmPC authoring – drafting de-novo or generic SmPCs against SAHPRA templates and guidelines.
Regulatory gap analysis – identifying wording misalignments and reference to approved Reference materials.
Variation strategy – classifying the variations and preparing the application and submission package
Life-cycle maintenance – continuous surveillance of literature and safety data to trigger timely updates.
Frequently Asked Questions (FAQ)
What is the legal status of an SmPC?
It is part of the marketing authorisation and its wording is legally binding.
Do SAHPRA and EMA use the same headings?
Yes – SAHPRA mirrors the EU QRD headings with minor local tweaks.
When is a Type II variation required?
For major safety or efficacy updates, new indications, posology changes, or new safety and efficacy data.
How long does a Type IB variation take?
30 + 7 working days clock-in-hand, plus clock-stops if questions arise.
Can marketing claims exceed the SmPC?
No – promotional materials cannot go beyond the SmPC wording.
How does MC Pharma support SmPC updates?
We classify the variation, draft the updated text, submit through e-submission portals and track approval.
Is the SmPC the same as the patient leaflet?
No – the SmPC is for healthcare professionals; the PIL is derived from it for patients.
References
European Medicines Agency. (2025). Product-information (QRD) templates – draft v11. (ema.europa.eu)
European Medicines Agency. (2023). Electronic product information (ePI) pilot page. (ema.europa.eu)
European Medicines Agency. (2024). ePI pilot report – lessons learned. (ema.europa.eu)
European Medicines Agency. (2024). Assessment guide for SmPC Section 5.1. (ema.europa.eu)
European Commission. (2024). Draft Variations Classification Guideline. (health.ec.europa.eu)
European Commission. (2009). Guideline on the SmPC (EudraLex Vol 2C). (health.ec.europa.eu)
Directive 2001/83/EC of the European Parliament and Council. (2001). Community code relating to medicinal products for human use. (eur-lex.europa.eu)
Medicines and Healthcare Products Regulatory Agency. (2020). Best-practice guidance on labelling & packaging. (assets.publishing.service.gov.uk)
GOV.UK. (2024). Medicines: Packaging, labelling and PIL (MHRA overview). (gov.uk)
Royal Pharmaceutical Society. (2025). Overview of SmPCs for trainee pharmacists.
SAHPRA. (2022). Clinical Evaluation Guideline (SmPC equivalent). (sahpra.org.za)
SAHPRA / SADC. (2019). Draft Guideline on Product-Information & Labelling. (sahpra.org.za)
World Health Organization. (2024). Contents & structure of a WHOPAR. (extranet.who.int)
EMA PLM Portal. (2024). ePI user guide for applicants. (plm-portal.ema.europa.eu)
Need a gap analysis or XML conversion for your next SmPC submission?
Why choose MC Pharma?
With over 26 years of experience in navigating South Africa’s complex regulatory landscape, MC Pharma is your strategic partner for patient safety, regulatory excellence, and market success.





Comments